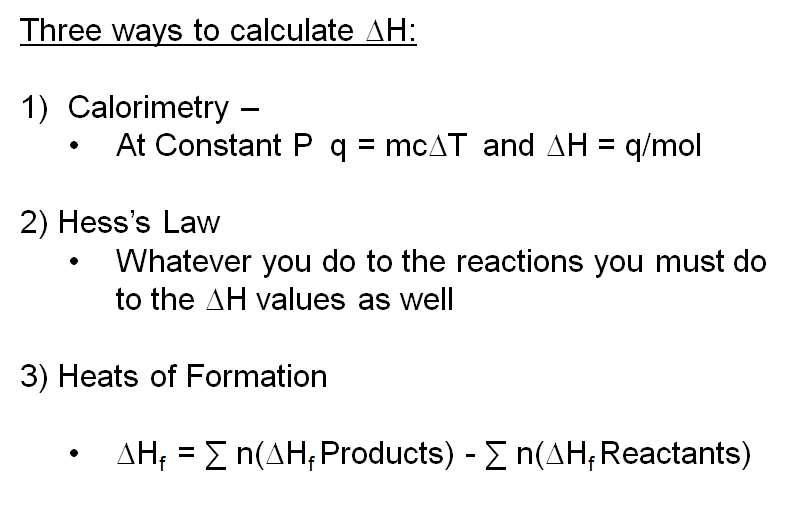
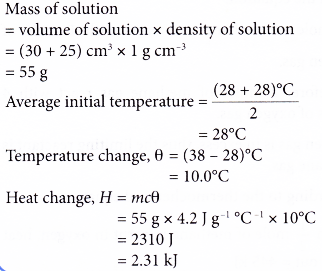
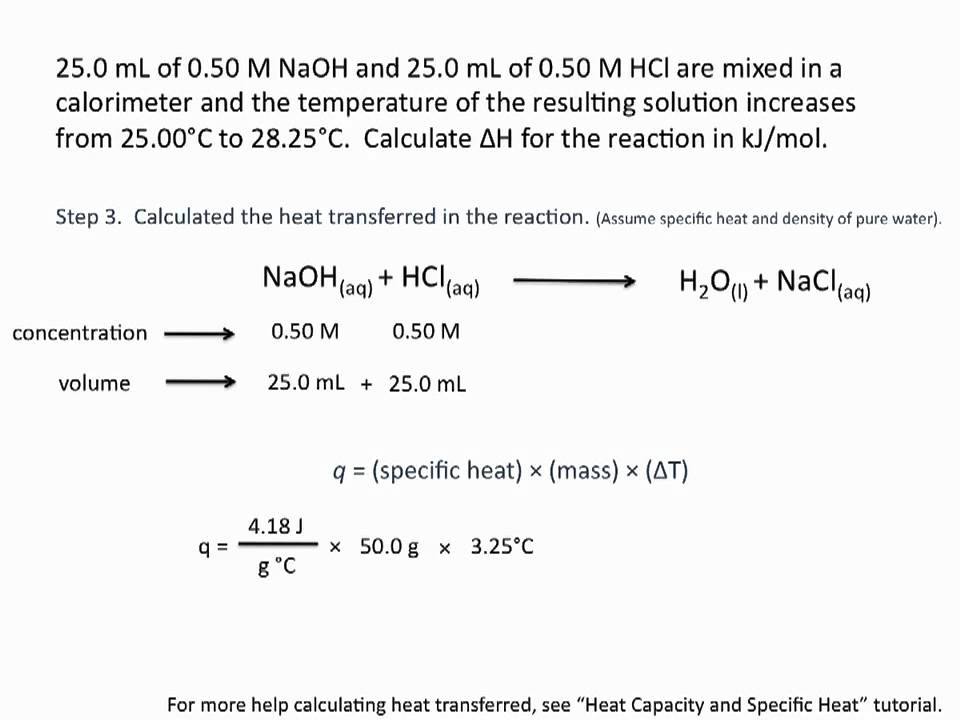
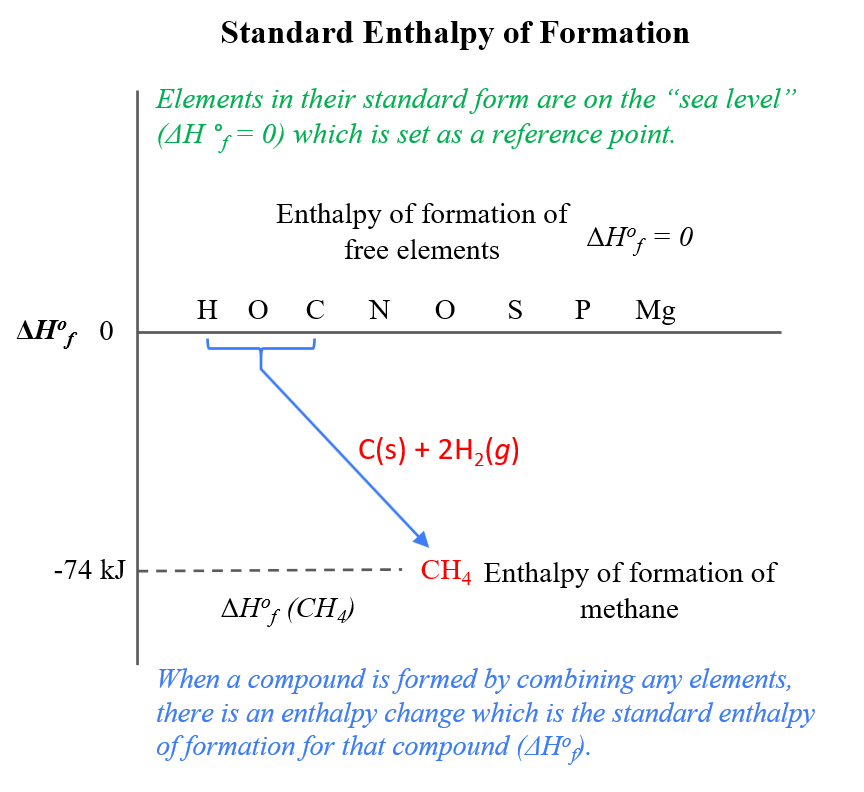
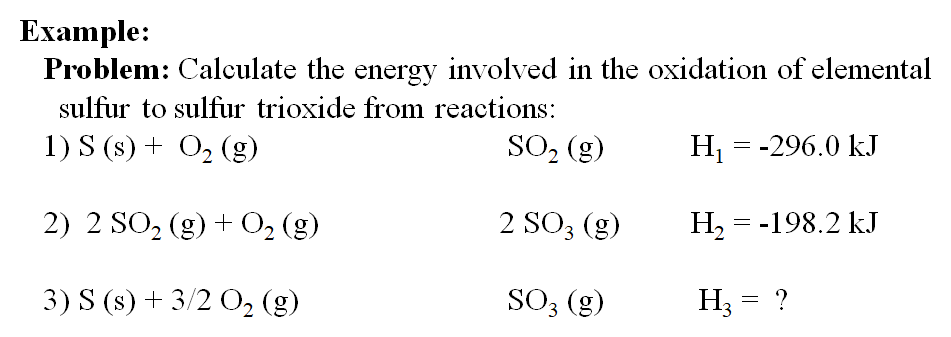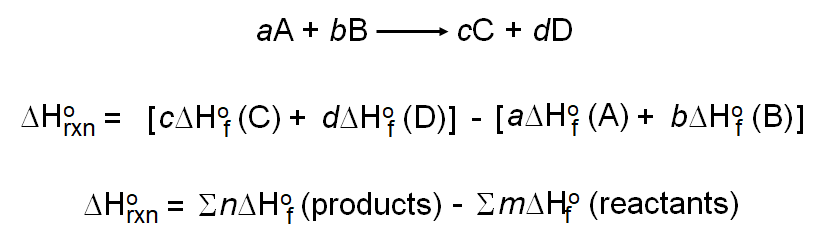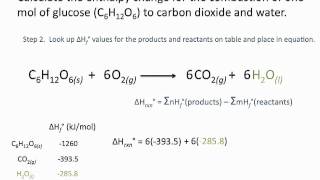Question Video: Calculating the Enthalpy Change for the Reaction between Phenol and Diatomic Hydrogen Using Standard Enthalpies of Combustion | Nagwa

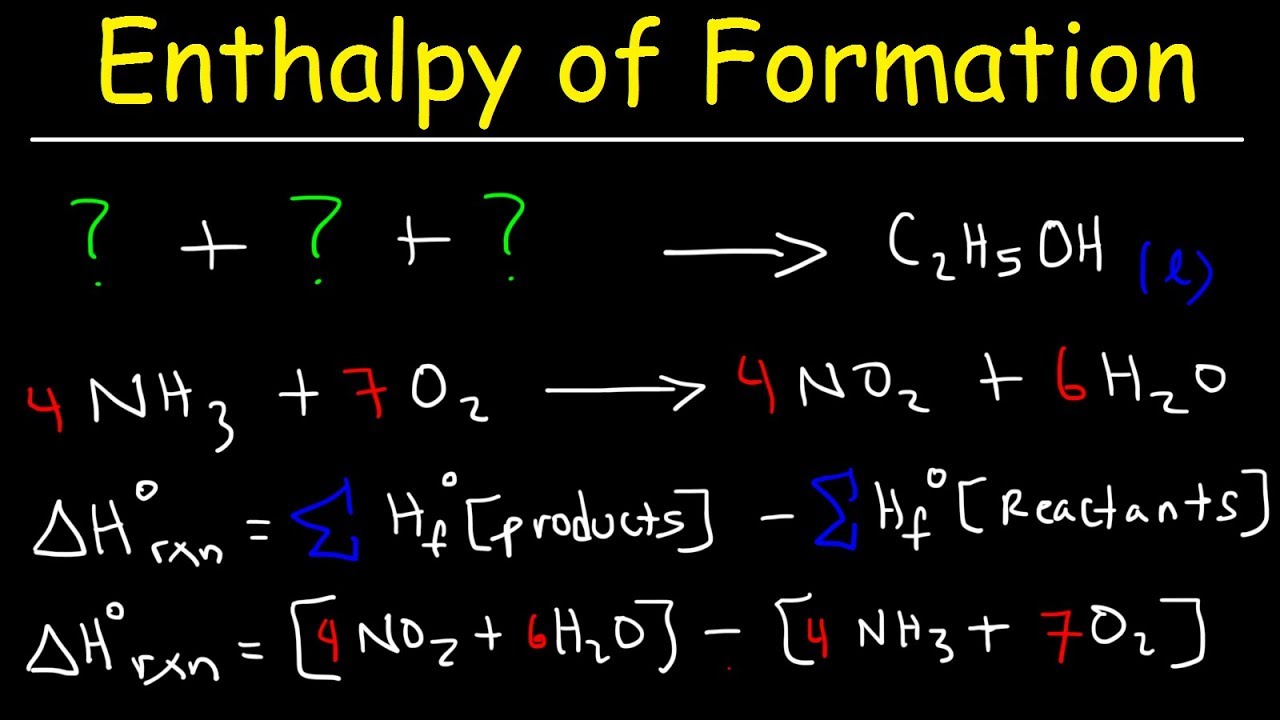
Calculate the Standard Enthalpy of the Reaction,From the Following δH° Values - Chemistry | Shaalaa.com

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (
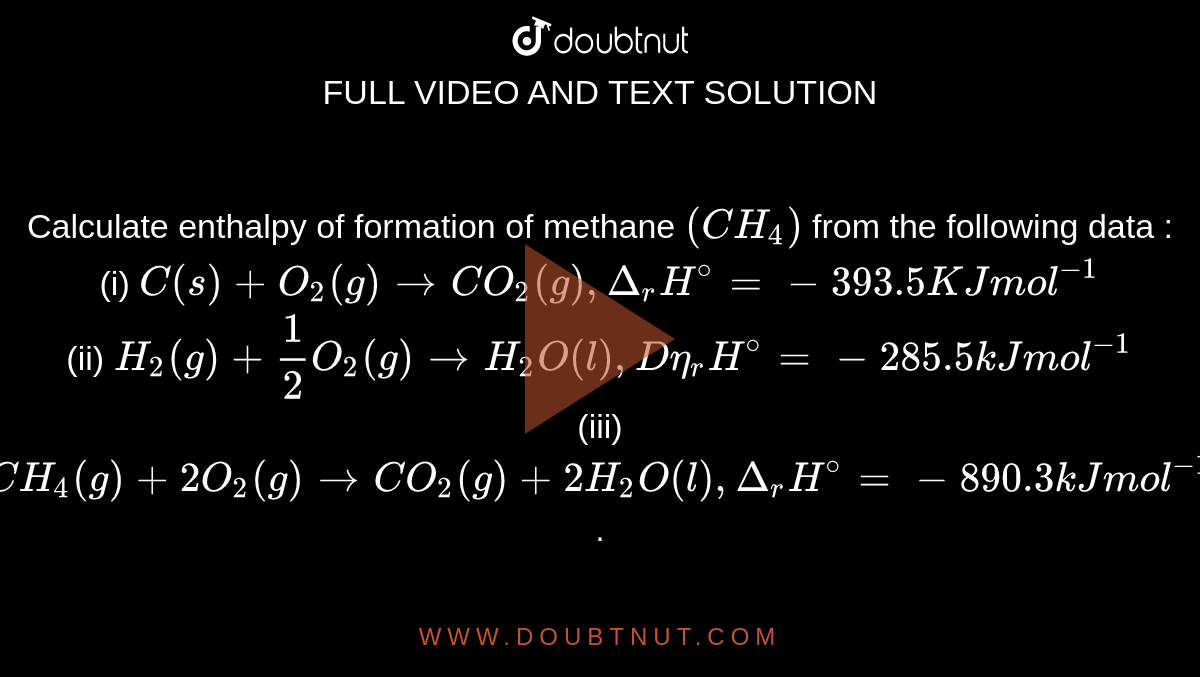
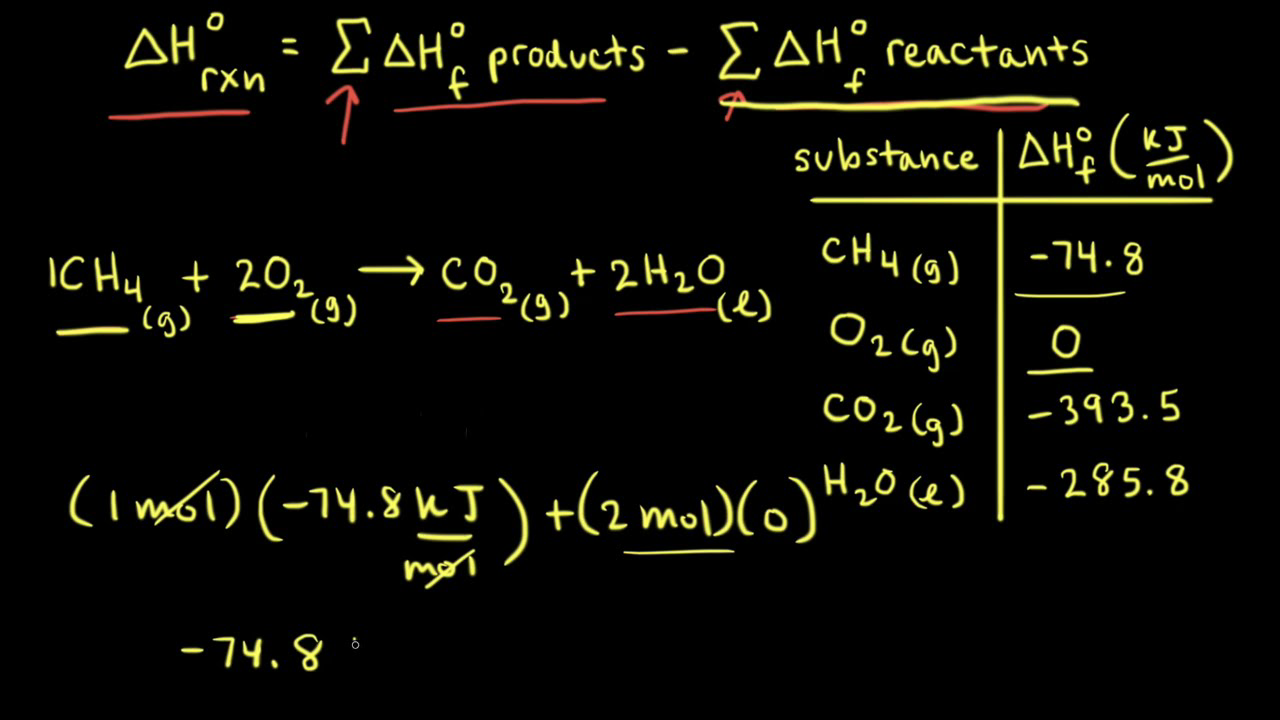
Calculate enthalpy of formation of methane (CH4) from the following data : (i) C(s) + O(2)(g) to CO(2) (g) , DeltarH^(@) = -393.5 KJ mol^(-1) (ii) H2(g) + 1/2 O(2)(g) to H(2)O(l) ,